Genetic cardiomyopathy is a silent hereditary condition that affects the heart muscle’s strength, structure, and electrical activity. Unlike heart diseases that stem from lifestyle habits or external triggers, this disorder originates from within—the DNA itself. Passed down through generations, it alters how the heart functions, sometimes without any visible symptoms until complications arise.
This form of cardiomyopathy is more than a medical term; it represents a family legacy woven into genetic material. For many, its presence goes unnoticed until a routine scan or a sudden cardiac event reveals the underlying issue.
How the Genetics Play a Role
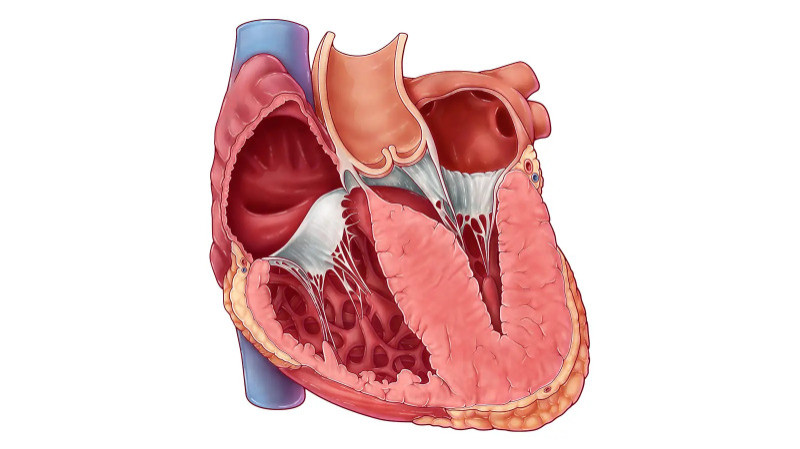
The human heart is powered by an intricate network of proteins, many of which are encoded by specific genes. When one or more of these genes undergo mutation, the resulting proteins may not function as they should, weakening heart muscle fibers or disrupting electrical signaling. These changes can gradually or suddenly impair the heart’s ability to pump blood efficiently.
Inherited mutations are typically passed in a dominant fashion, meaning only one faulty gene from either parent can cause the condition. In other scenarios, recessive or X-linked patterns of inheritance are responsible. The risk of transmission to the next generation depends heavily on the genetic pattern involved.
Forms of Genetic Cardiomyopathy
Genetic cardiomyopathy isn't a single disease but a spectrum of disorders with varying presentations. The most recognized types include:
Hypertrophic Cardiomyopathy (HCM): This is the most prevalent inherited heart condition, marked by abnormal thickening of the heart muscle. The thickened walls can block blood flow, leading to symptoms like breathlessness, palpitations, or even fainting. In some individuals, it remains asymptomatic but can be detected during routine evaluations.
Dilated Cardiomyopathy (DCM): In DCM, the heart chambers—especially the left ventricle—become stretched and thin, reducing the heart’s ability to contract and circulate blood effectively. Genetic forms of DCM often appear in adolescence or adulthood and may be confused with lifestyle-related heart failure.
Arrhythmogenic Cardiomyopathy (ACM): Formerly called ARVC, this condition causes the muscle tissue of the heart to be replaced by fat or fibrous tissue, particularly in the right ventricle. It disrupts normal electrical signaling and increases the risk of arrhythmias or sudden cardiac death, especially during physical exertion.
Left Ventricular Noncompaction (LVNC): A lesser-known form, LVNC occurs when the muscle wall of the left ventricle develops a spongy appearance due to incomplete compaction during fetal development. Though rare, its genetic links are becoming more understood with advanced imaging and sequencing tools.
What Increases the Risk?
The presence of a gene mutation does not always result in disease. Some individuals remain unaffected carriers, while others develop mild to severe symptoms. Several factors may influence disease expression, including other modifying genes, environmental exposures, or lifestyle.
The most significant risk, however, lies in family history. If a parent or sibling is diagnosed with cardiomyopathy, there’s a higher likelihood of inherited susceptibility. Other risk factors include early-onset heart failure, sudden unexplained deaths in the family, or fainting episodes without clear cause.
When and How to Spot the Condition
Symptoms can appear gradually or emerge suddenly. Common indicators include fatigue, shortness of breath, chest discomfort, or irregular heartbeats. In many families, the condition is only uncovered after a young family member suffers a sudden cardiac event.
Diagnosis usually begins with clinical evaluations like echocardiograms or ECGs, but genetic testing has transformed how early and accurately this condition is identified. Testing not only confirms the presence of mutations but also helps map risk across generations. Genetic counseling becomes essential here, offering families clarity and guidance on further steps.
Treatment Pathways and Lifestyle Modifications
There is no universal cure for genetic cardiomyopathy, but its symptoms and complications can be managed effectively. Treatment varies based on the specific type and severity. Common approaches include:
Medications such as beta-blockers, calcium channel blockers, or anti-arrhythmic drugs to regulate heart rhythm and reduce strain
Implantable cardioverter defibrillators (ICDs) in high-risk individuals to prevent sudden cardiac arrest
Lifestyle changes, such as avoiding high-intensity sports or stimulants
Monitoring for complications like heart failure or clot formation
In advanced cases, particularly when the heart’s function declines significantly, heart transplantation may be the only viable long-term solution.
Looking Ahead: The Power of Prevention and Family Screening
Understanding genetic cardiomyopathy is not just about treating a disease—it’s about proactive family health. Early detection through family screening, especially for those with known genetic variants, can prevent life-threatening outcomes.
Advancements in genetic research and precision medicine are providing new hope. Targeted therapies, gene editing, and improved surveillance methods are on the horizon, promising better management for future generations.
Conclusion
Genetic cardiomyopathy represents a profound link between family heritage and heart health. By identifying the condition early and managing it through modern interventions, individuals and families can live fuller, safer lives. Though invisible at birth, its presence can be unveiled with awareness, timely testing, and coordinated care. The heart, after all, carries not just life—but legacy.